IL2/IL15 targets in tumor immunotherapy

The homeostasis of the immune system relies on innate and adaptive immune responses, and the realization of these responses is inseparable from the fine regulation of a series of cytokines. Among them, the cytokine receptor γ chain family is one of the most widely studied cytokines. As important members of the γ chain family, the IL-2 and IL-15 pathways play a key role in the regulation of immune system functions.
In recent years, research on these two cytokines has been continuously deepened, gradually revealing their unique mechanisms and application potential in tumor treatment and autoimmune diseases. From the classic high-dose IL-2 therapy to the development of new IL-2 and IL-15 derived drugs, this field is undergoing an important transition from "classic mechanisms" to "frontier exploration".
How do IL-2 and IL-15 function in the body?
IL-2 and IL-15 are Type I four α-helix bundle cytokines, which have many common biological activities, including stimulating the proliferation and activation of T cells and NK cells, inducing B cell immunoglobulin synthesis, and supporting the differentiation of cytotoxic effector cells.
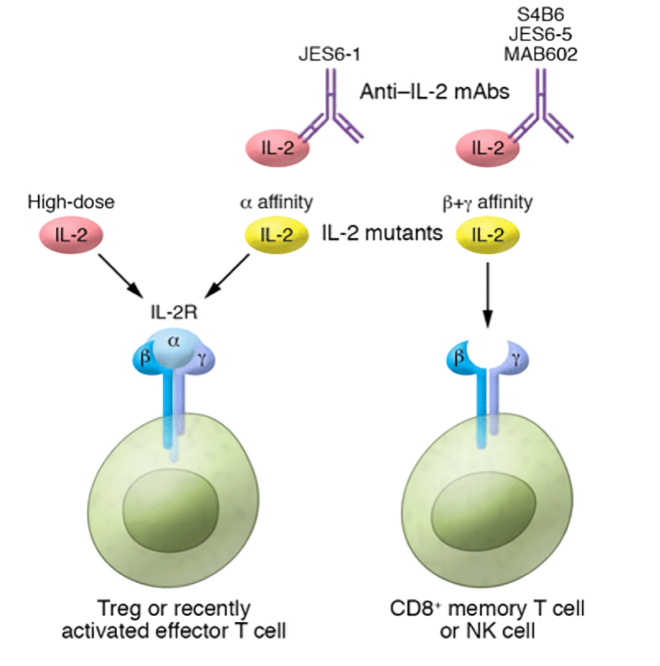
Figure 1. IL-2 and its receptor signal transduction
IL-2 is mainly secreted by activated T cells, and it activates the JAK-STAT, PI3K/AKT, and MAPK/ERK signaling pathways by binding to its three-part receptor (IL-2Rα/CD25, IL-2Rβ/CD122, IL-2Rγ/CD123), promoting the proliferation and functional enhancement of T cells and NK cells. While IL-2 activates effector T cells, it can also significantly expand regulatory T cells (Treg), thus achieving a dynamic balance between immune activation and suppression [1]. Low-dose IL-2 preferentially binds to the high-affinity IL-2Rβγ receptor to activate Treg cells, which can significantly improve the immune tolerance of patients with autoimmune diseases. High-dose IL-2, after saturating Treg, will bind to receptors with medium affinity, enhancing the cytotoxicity of CD8+ T cells and the killing function of NK cells, hence the antitumor effect of IL-2 has significant dose dependence.
In contrast, IL-15 is secreted by monocytes and macrophages, and its signal transmission depends on the presentation of IL-15Rα. IL-15 shares IL-2Rβ and the γ chain with IL-2, and the signaling pathways are highly similar, but there are significant differences in biological characteristics. IL-15 is more focused on enhancing the activity and function of memory CD8+ T cells, while also activating the killing ability of NK cells, and its effect on Treg cells is relatively weak [1]. Unlike IL-2, IL-15 can maintain the activity of effector cells in long-term immune surveillance, especially showing significant advantages in the treatment of tumor immunity and infectious diseases. In addition, IL-15 has the characteristic of low toxicity, which makes it an ideal candidate for the development of long-acting immunotherapeutic drugs.
Although IL-2 and IL-15 are highly similar in receptor structure and signaling pathways, they are complementary in function and application. IL-2 can be used at high doses to activate antitumor immunity, or at low doses to expand Treg cells for the treatment of autoimmune diseases; while IL-15 is more inclined to enhance the function of effector cells, and is suitable for tumor immunotherapy that requires persistent immune activation [3]. In recent years, with the development of bioengineering technology, innovative drugs targeting IL-2 and IL-15 are rapidly advancing, providing more possibilities for precise immune regulation in tumor treatment and autoimmune diseases.
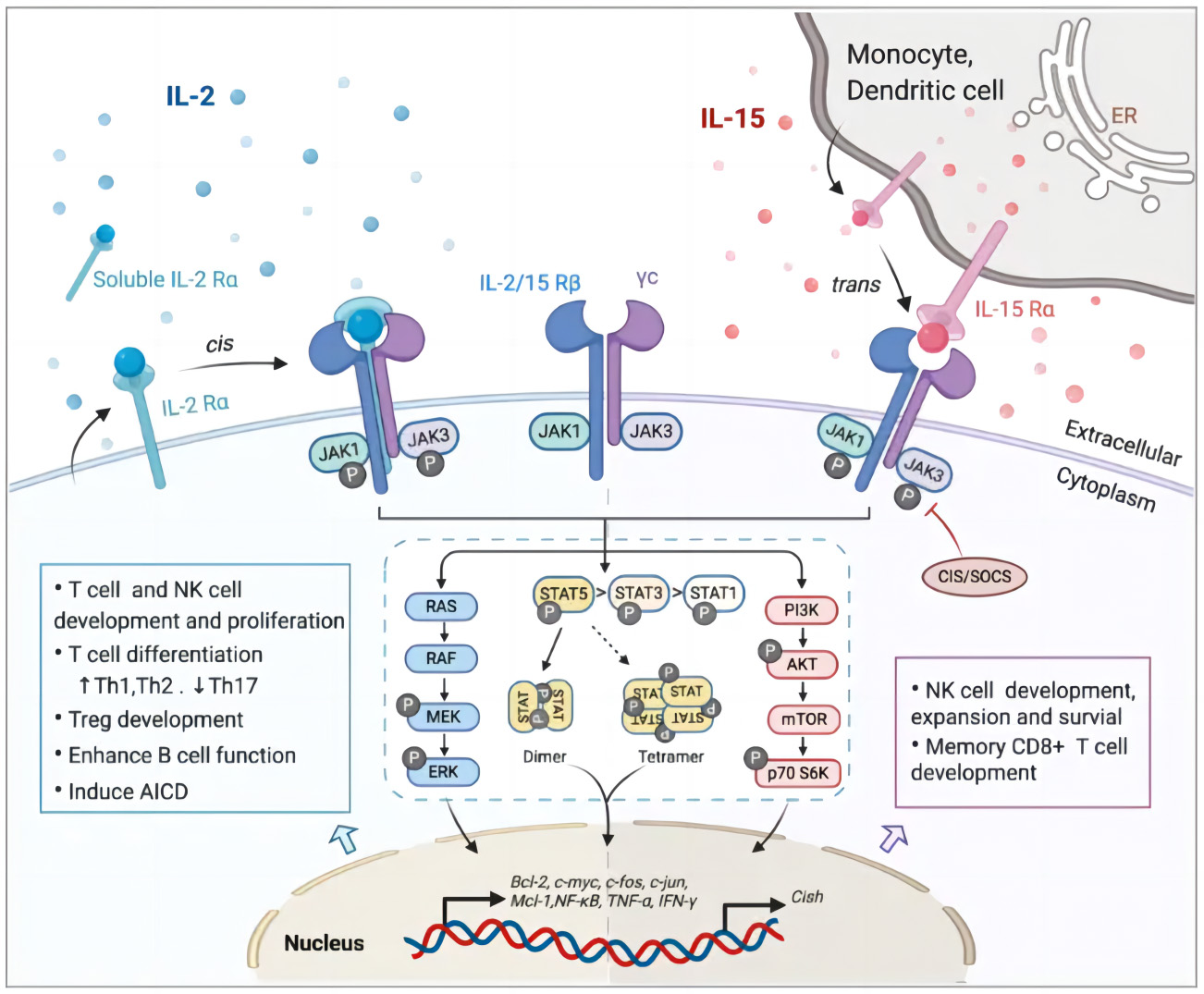
Figure 2. IL-2 and IL-15 signaling pathways
IL-2 and IL-15 Related Indications
Targeting IL-2
Tumor immunotherapy (high-dose IL-2): Melanoma, Renal Cell Carcinoma (RCC)
Autoimmune diseases (low-dose IL-2): Systemic Lupus Erythematosus (SLE), Inflammatory Bowel Disease (IBD), Type 1 Diabetes, Graft-versus-Host Disease (GVHD)
Targeting IL-15
Tumor immunotherapy: Leukemia and lymphoma, solid tumors (such as non-small cell lung cancer, melanoma, renal cell carcinoma), enhancer for CAR-T cell therapy
Infectious diseases: Chronic viral infections (HIV and HCV)
Autoimmune diseases: Rheumatoid arthritis, autoimmune diabetes, inflammatory bowel disease, celiac disease, and psoriasis
Vaccine adjuvant
[1] Yang Y, Lundqvist A. Immunomodulatory Effects of IL-2 and IL-15; Implications for Cancer Immunotherapy. Cancers (Basel). 2020;12(12):3586. Published 2020 Nov 30. doi:10.3390/cancers12123586
[2] Holcomb EA, Zou W. A forced marriage of IL-2 and PD-1 antibody nurtures tumor-infiltrating T cells. J Clin Invest.2022;132(3):e156628. doi:10.1172/JCI156628
[3] Waldmann TA. The shared and contrasting roles of IL2 and IL15 in the life and death of normal and neoplastic lymphocytes: implications for cancer therapy. Cancer Immunol Res. 2015;3(3):219-227. doi:10.1158/2326-6066.CIR-15-0009
Related Targets
| IL-2 Rα | IL-2 Rβ | IL-15 Rα | IL-15 Rβ |
| JAK1 | JAK3 | STAT | PI3K |
